Reactivity on cold Surfaces
Publié le 27 septembre 2014 par , , , .
Team
Francois Dulieu (Prof – Team Leader), Saoud Baouche (Engineer), Henda Chaabouni (Ass. Prof.), Vincent Cobut (Ass. Prof.), Emanule Congiu (Ass. Prof.), Stéphane Diana (Engineer) , Francois Lachèvre (Tech.), Henri Lemaître (PhD student), Audrey Moudens (Ass. Prof.), Thanh Nguyen (PhD student).
Context : How molecules are formed at the surface of cold grains ?
The molecules (H2O, CO2...) existed well before the birth of our Earth. Radioastronomy is able to decipher this chemical history, when the molecules are in the gas phase. Unfortunately, the molecular complexity remains almost invisible, as complex molecules are synthesized and frozen on the surface of cold dust particles. Therefore, only laboratory astrophysics can explore this micro world. Despite all the recent observational progress, the enigma of astrochemistry is still unresolved and this is why we built state-of-the-art surface science apparatus.
The team « Reactivity on cold surfaces » is mostly devoted to experimental physics. It is located at the Cergy-Pontoise University. We study the evolution of atoms and molecules on surfaces relevant to astrophysics. We are interested in reactivity but also in all related processes like sticking, diffusion and desorption. We use atomic and molecular beams targeted on surfaces (graphite, silicates, ices...) cooled down to 6K, in order to mimic the extreme conditions of star forming regions.
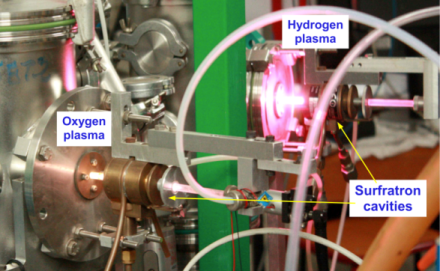
Experimental set-up
We mostly use two complementary set-up for our studies.
- FORMOLISM – Developped since 2001.
UHV
2 atomic or molecular beams (H, N, O, CO, NO, H2CO…). Project : nanograins source (Coronene).
Surfaces : removable sample (graphite, gold, silicate) and an in-situ controlled water ice growing system (amorphous, porous, crystalline…).
Surface temperature control : 6-300K, project 10-800K.
Detection tools :
Mass spectrometry (its use is fourfold) : Beam compositions, During Exposure Detection, Thermally Programmed Desorption, Internal energy of atoms or molecules.
Reflection Absorption Infra Red Spectroscopy.
Laser system (REMPI 2+1) coupled with a time of flight detection.
- VENUS – developed since 2011

Up to 5 atomic or molecular beams. Only 2 presently running.
Surfaces : Rotatable sample holder with 3 surfaces.
Temperature Range : 10-300K
Mass spectrometry (its use is fourfold) : Beam compositions, During Exposure Detection, Thermally Programmed Desorption, Internal energy of atoms or molecules.
Reflection Absorption Infra Red Spectroscopy.
Recent studies
- Molecular synthesis : Molecular synthesis : H2O (Chaabouni et al 2012), NH2OH (Congiu et al 2012), Nitrogen oxides (Minissale et al 2013, 2014), CO2 (Noble et al 2011, Minissale et al 2012,2014)…
- Diffusion and desorption of oxygen : Diffusion is faster than expected at low temperature (<10K) (Minissale et al 2013,2014, Congiu et al 2014), but desorption energy is larger than previously estimated (Minissale et al submitted).
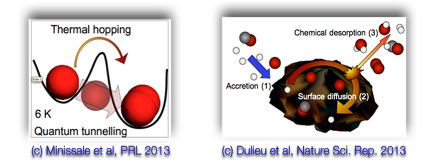
- Chemical desorption : Experimental evidence (Dulieu et al 2013, Minissale&Dulieu 2014) :
It is an important step linking the solid-state chemistry and observations of the gas phase.
- Thermal desorption : Role and importance of surface coverage and surface type in sub-monolayer regime (Noble et al 2012a,b).
- Water ice morphology : After its synthesis or after H recombination, water ice is compact or compacted : (Accolla et al 2012, 2013)